MARKETS WE SERVE
Stelioz Solutions Inc. strives to provide clients a bridge between industry regulations and market success. Our goal is to support people with strategic, timely and creative services for their businesses, and make their ideas come to life, from formulation to marketing.
- Over 800 NPN licenses applied and approved (over 350 Class III NPNs successfully obtained)
- Consulted and supported over 80 brands- from formulation to market launch and beyond
- Provided regulatory support, quality management, site and documentation audit to sites (manufacturing, warehousing, importing of NHPs and dietary supplements) across Canada

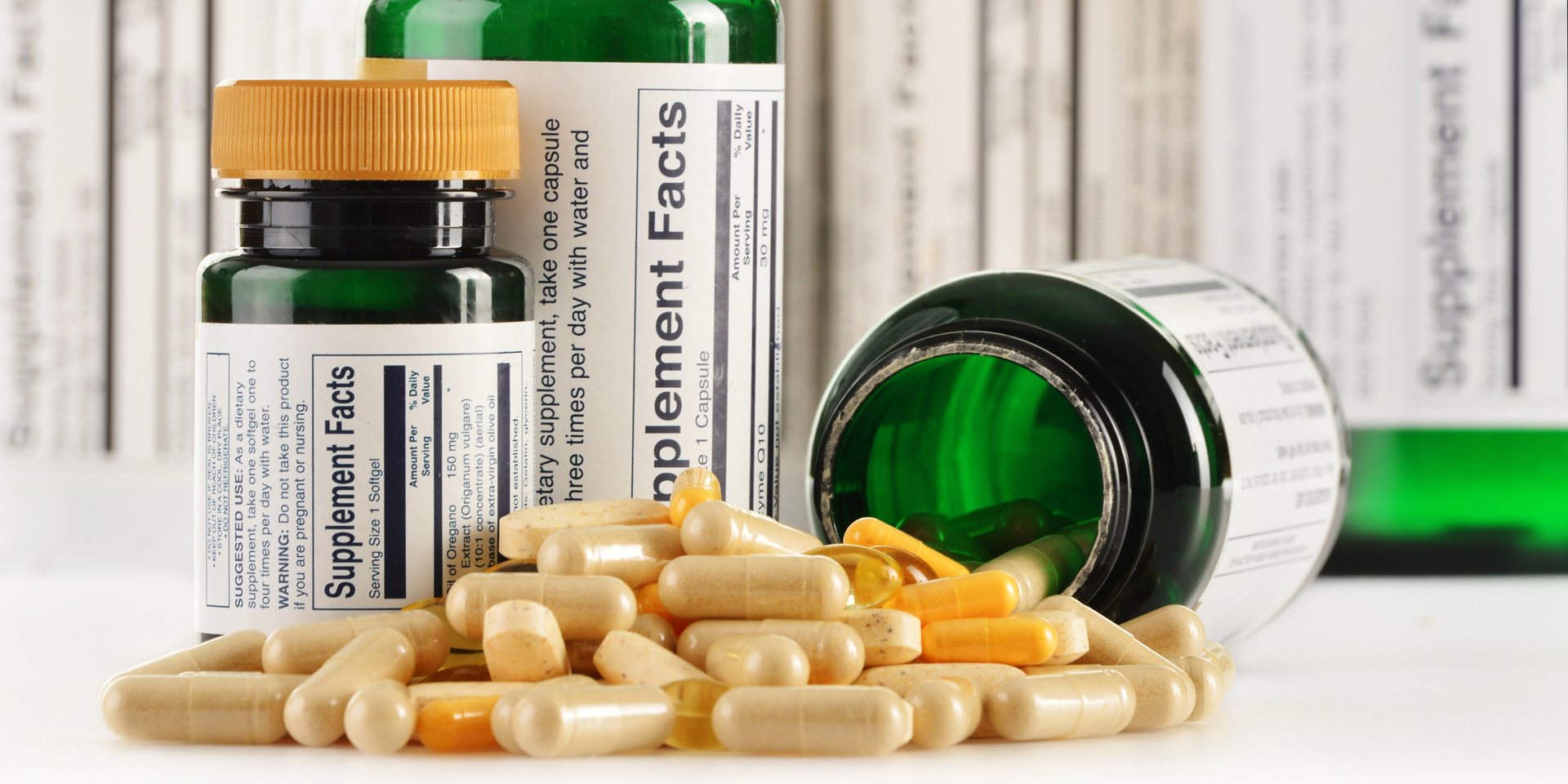
Natural Health Products and Dietary Supplements
- Formulation, product development, consultation for lab testing for finished product and stability
- Canadian regulatory compliance (NPN, Master file, NHP label compliance)
- US regulatory support (Label preparation and compliance, FDA food facility registration, claim substantiation, structure function claim notification)
- Quality management from formulation to post marketing compliance
- Manufacturing and warehousing site compliance (site licensing, FSRN, audit, employee training, SOP development)

Foods, Beverages and Supplemented Foods
- Supplemented Food regulations support
- Product development, safety and quality systems review and audit
- GMP compliance, HACCP and PCP development
- SFCR licensing, Temporary Market Authorization (TMA), Novel Food Notification
- Label preparation and compliance
- New Dietary Ingredient notification (NDIN) and GRAS notification

Cosmetics and Personal Care
- Formulation, product development and testing consultation
- Cosmetic notification (CNF) and amendments
- Label preparation and compliance
- Quality management from formulation to post marketing compliance

Pet Supplements and Veterinary Health Products
- Formulation, product development and testing consultation
- VHP notifications for Notification Number (NN)
- New substance addition to ‘List of Permitted Substances’ application
- Label preparation and compliance
- Post marketing compliance

CALL US @
1 866-846-7047
647-558-4602
OR
Schedule a Free Consultation